Abstract
Background: Multiple myeloma (MM) is known to be a heterogeneous disease encompassing several molecular subtypes that can be defined either by means of genetic or molecular testing. In the multigene tests, the 'PR' subtype enriched with proliferation-related genes has a substantial impact on the prediction of the risk of MM recurrence. Ki-67 is a nuclear protein involved in cell cycle regulation and is considered an indicator of proliferation. It is present in dividing cells during all active phases of the cell cycle (S, G1, G2 and mitosis phases) and is distinctly absent in resting or quiescent (G0) cells. Ki67 expression has been widely used as an index to evaluate the proliferative activity of breast cancer and lymphoma but the impact of Ki-67 expression on the clinical outcomes of patients with MM has largely been unexplored. This study aimed to investigate whether Ki-67 expression by IHC is an indicator of outcome in MM patients, especially those treated with novel therapies combined with or without autologous stem cell transplantation.
Methods: The MM database was interrogated from January 2012-May 2017 for all newly diagnosed symptomatic MM subjects with Ki67 expression levels estimated by IHC on bone marrow biopsy specimens at diagnosis. Clinical features and outcomes were captured and compared between the cohorts of Ki67 high (Ki67-hi) subjects (Ki67 >5%) and Ki67 low (Ki67-lo) subjects (Ki67 ≤5%). Continuous variables were compared using median two sample tests, while incidences and proportions were compared using Fisher's exact tests. Survival distributions were estimated with Kaplan Meier techniques and compared between Ki67 cohorts using log-rank tests. Differences in overall survival (OS) and progression free survival (PFS) between the positive and negative cohorts were quantified with hazard ratios from Cox regression. Model selection was performed to determine adjusted hazard ratios
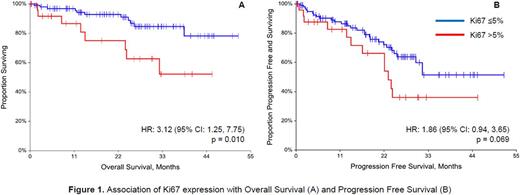
Results: A total of 125 MM subjects were identified with Ki67 values at diagnosis (26 Ki67-hi, 99 Ki67-lo). Gender, race, and age were similar between the two cohorts. There were no significant differences in beta-2 microglobulin (B2M) or LDH levels between the cohorts (p=0.236 and p>0.999, respectively); however, the median B2M in the Ki67-hi cohort was numerically higher than in the Ki67-lo cohort (4.6 vs 3.5 mg/L). A significantly greater proportion of Ki67-lo subjects had good risk cytogenetics (58.3%) compared to those who were Ki67-hi subjects (32.0%, p=0.035). There were no significant differences in the tested flow cytometry immunophenotype markers except for CD38, which was expressed in 87.9% of the Ki67-lo cohort compared to 53.9% of the Ki67-hi cohort (p<0.001). OS was significantly worse in the Ki67-hi cohort (HR: 3.12, 95% CI: 1.25, 7.75, p=0.010). Additionally, after adjusting for age and adverse risk, the risk of death in the Ki67-hi cohort is 4 times that of the Ki67-lo cohort (p=0.004, 95%CI: 1.58, 10.29). PFS was worse in the Ki67-hi cohort (HR: 1.86; 95% CI: 0.94, 3.65; p=0.069). Median follow-up time was 21.9 months (IQR 9.7 - 30.7 months).
Conclusions: Our early results demonstrate that elevated Ki67 expression measured by IHC staining is associated with worse OS in newly diagnosed MM. IHC staining for Ki67 can potentially be utilized in economically constrained healthcare settings globally. Our current work focuses on standardization of Ki67 assessment and specification of its role as a dynamic biomarker of the treatment effectiveness in newly diagnosed MM.
Voorhees: Amgen: Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Oncopeptides: Consultancy; Novartis: Consultancy; Takeda: Consultancy; Bristol-Myers Squibb: Consultancy; Celgene: Consultancy, Speakers Bureau. Usmani: Array BioPharma: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Skyline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Onyx: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau; Novartis: Speakers Bureau; Millennium: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Honoraria, Research Funding. Bhutani: Amgen: Speakers Bureau; Prothena Therapeutics: Research Funding; BMS: Speakers Bureau; Takeda Oncology: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal